Tactical collaboration for execution on the field
Aenean lacinia bibendum nulla sed consectetur. Maecenas faucibus mollis interdum. Nulla vitae elit libero, a pharetra augue. Vivamus sagittis lacus vel augue laoreet rutrum faucibu.
- Posuere erat a ante venenatis
- Praesent commodo cursus
- Posuere erat a ante venenatis
Your top 5 tactical needs
Your top 5 tactical needs
Enhancing the probability to get reimbursement with a premium price in due time
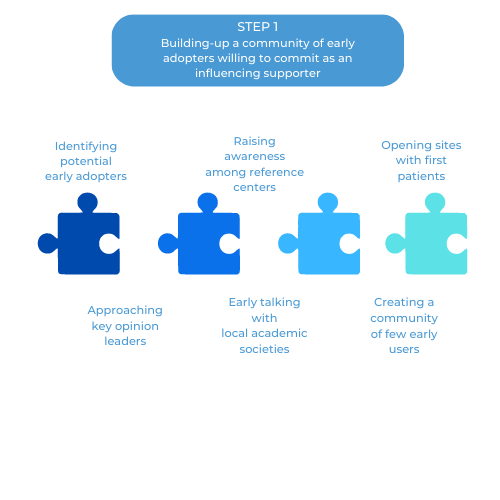
Building-up a community of early adopters willing to commit as an influencing supporter
Optimizing the timeframe of reimbursement submission to setup a commercial pre-launch
Planning the full commercial scale-up by assessing the best sales/marketing option : local distribution vs direct sales team
Keeping control and agility without fix (heavy) costs and with full flexibility to accelerate and to slowdown whenever it is needed
Why Caredis now?
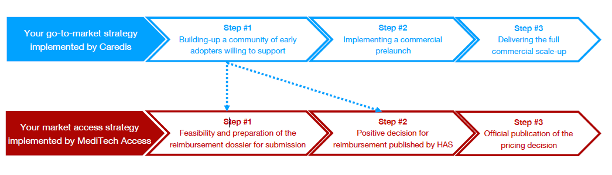
Caredis works on the field and in full alignement with the market access strategy (MediTech Access) by dealing with kols, reference centers and academic stakeholders to raise support for reimbursement
However strong the clinical dossier is, French healthcare authorities are used to asking feedback to French early users in the process of making the decision about reimbursement
For innovative technologies, Caredis can trigger local funding in university hospitals to purchase devices awaiting reimbursement
Caredis helps to implement the best setup by identifying the potential partners / profiles at early stage in order to kickoff just after the announcement of reimbursement
Caredis’s compensation is a combo of fix fees, time fees or success fees depending on the nature of activities and their volume whenever requested by the Client. Monthly retainer fees can be an option
At this early stage of market penetration, a full time employee based in France is NOT cost effective
Full alignment and collaboration
« Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed non risus. Suspendisse lectus tortor, dignissim sit amet, adipiscing nec, ultricies sed, dolor. Cras elementum ultrices diam. Maecenas ligula massa, varius a, semper congue, euismod non, mi. Proin porttitor, orci nec nonummy molestie, enim est eleifend mi, non fermentum diam nisl sit amet erat. Duis semper. Duis arcu massa, scelerisque vitae, consequat in, pretium a, enim. Pellentesque congue. Ut in risus volutpat libero pharetra tempor. Cras vestibulum bibendum augue. Praesent egestas leo in pede. Praesent blandit odio eu enim. Pellentesque sed dui ut augue blandit sodales. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Aliquam nibh. Mauris ac mauris sed pede pellentesque fermentum. Maecenas adipiscing ante non diam sodales hen
Step by step roadmap
with potential milestones on the field
Full commercial launch
Step #3
Planning and delivering the full commercial scale-up
Brainstorming on go to market options : direct vs distribution
Scouting and hiring sales & clinical talents
Searching distributors with a track record
Managing third parties to set up a legal entity
Negociating a distribution contract
Step #2
Implementing a commercial pre-launch
Showing up in academic events (meetings, webinars)
Facilitating publications of local guidelines & protocols
Searching distributors with a track record
Searching distributors with a track record
Negociating a distribution contract
Negociating a distribution contract
Managing third parties to set up a legal entity
Managing third parties to set up a legal entity
Managing third parties to set up a legal entity
Step #1
Planning and delivering the full commercial scale-up
Brainstorming on go to market options : direct vs distribution
Searching distributors with a track record
Scouting and hiring sales & clinical talents
Negociating a distribution contract
Managing third parties to set up a legal entity